Sunday 04 May 2025
Scientists have long been fascinated by a type of protein called intrinsically disordered proteins (IDPs), which are found in many living organisms and play important roles in various biological processes. However, due to their unique structure, IDPs have proven difficult to study using traditional methods.
Recently, researchers made significant progress in understanding the behavior of IDPs by developing a new theoretical framework that allows them to map these proteins onto simpler, more well-understood systems. This breakthrough has far-reaching implications for our understanding of protein function and disease.
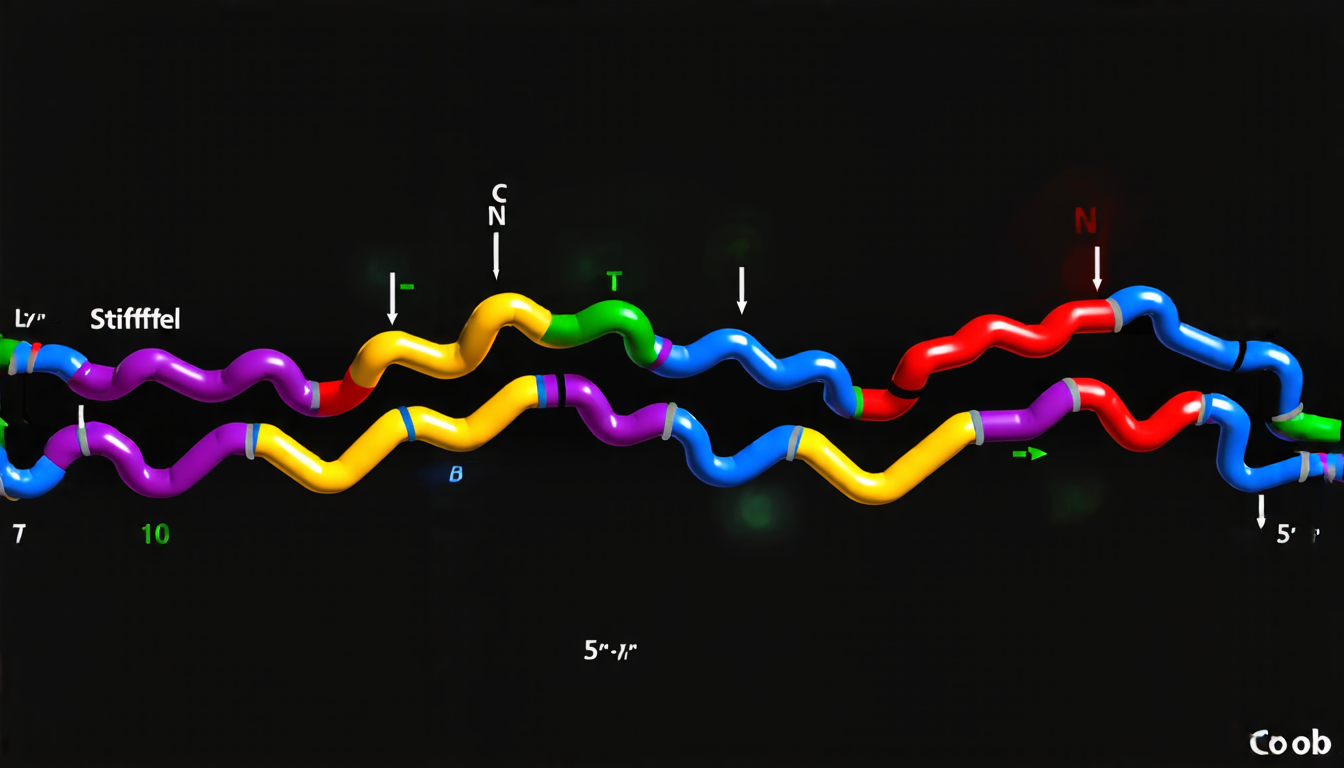
IDPs are unlike other proteins in that they lack a fixed three-dimensional structure. Instead, their molecular chains are highly flexible and can adopt different conformations depending on the environment and interactions with other molecules. This flexibility makes it challenging to predict how IDPs will behave and interact with other biomolecules.
To address this problem, researchers developed a new theoretical framework that treats IDPs as heterogeneous polymers, where the stiffness of each segment varies randomly. By using statistical mechanics techniques, they were able to derive an analytical expression for the effective persistence length of these polymers, which is a key parameter in determining their behavior.
The researchers then applied this framework to study the conformational properties of several IDPs and compared them with experimental data. They found that the theoretical predictions agreed remarkably well with the observed behavior, providing strong evidence for the validity of their approach.
This breakthrough has significant implications for our understanding of protein function and disease. By developing more sophisticated models of IDP behavior, researchers can gain insights into how these proteins interact with other biomolecules and contribute to various biological processes. This knowledge can be used to design new therapeutic strategies for diseases associated with IDPs, such as Alzheimer’s and Parkinson’s.
The development of this theoretical framework is a testament to the power of interdisciplinary research, combining advances in polymer physics, statistical mechanics, and computational biology. The authors’ findings demonstrate the importance of considering the complex behavior of IDPs when studying protein function and disease.
In the future, researchers plan to apply their new approach to study other types of proteins and biological systems, with potential applications in fields such as medicine, biotechnology, and materials science.
Cite this article: “Unlocking the Secrets of Intrinsically Disordered Proteins”, The Science Archive, 2025.
Intrinsically Disordered Proteins, Protein Structure, Statistical Mechanics, Polymer Physics, Computational Biology, Protein Function, Disease, Alzheimer’S, Parkinson’S, Biological Processes.