Saturday 06 September 2025
Scientists have long been fascinated by the intricate processes that govern self-assembly, the phenomenon where molecules come together to form complex structures without any external guidance. This process is essential for life, as it allows cells to create everything from proteins to membranes.
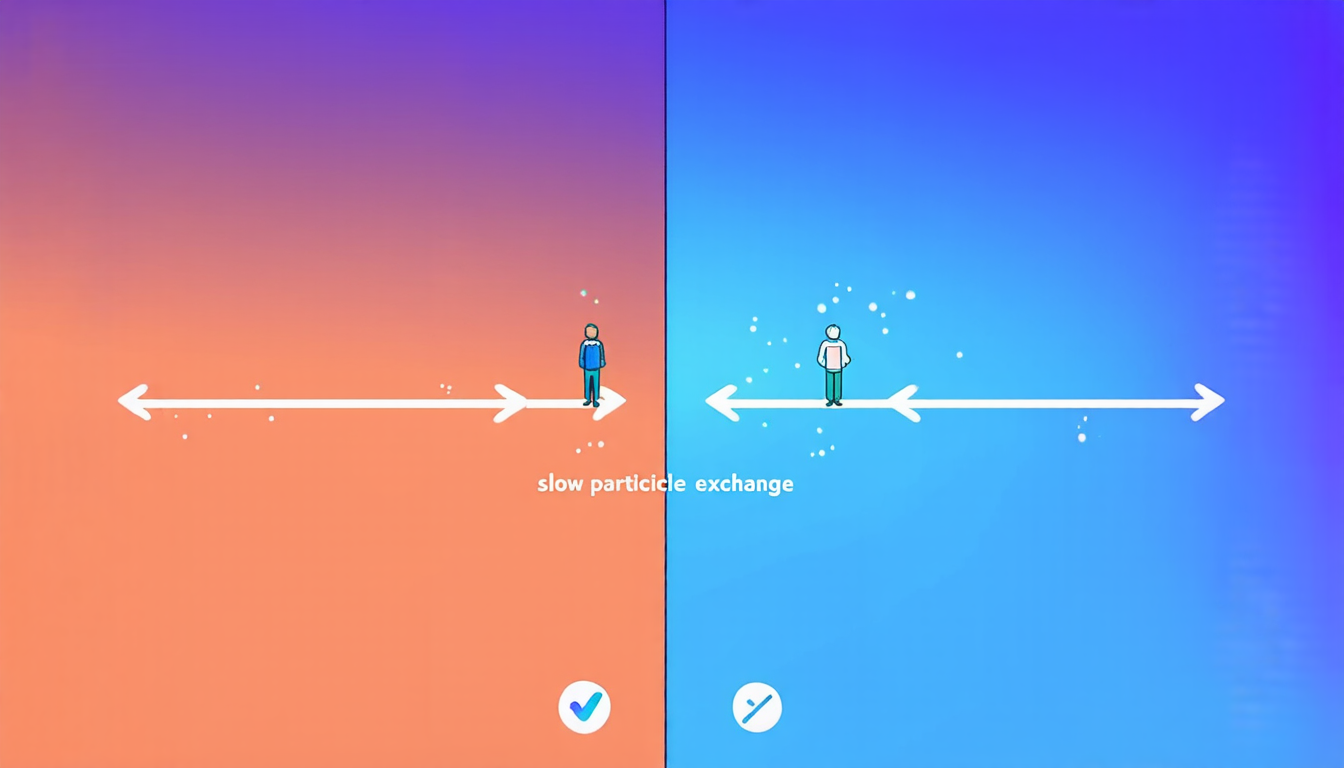
Recently, researchers made a breakthrough in understanding how slow particle exchange between different reaction domains can enhance self-assembly efficiency. This discovery has significant implications for our ability to design and control synthetic biomolecular systems, which could lead to innovative solutions for medicine, energy, and other fields.
To grasp the significance of this finding, let’s delve into the world of compartmentalized systems. In these systems, molecules are separated into different domains or compartments with distinct reaction rates and exchange dynamics. This setup allows researchers to mimic the complex interactions that occur within living cells, where different processes unfold at different timescales.
The study focused on a specific scenario where two compartments were connected by slow particle exchange. The researchers found that this slow exchange facilitated self-assembly by allowing the system to separate the formation of stable nuclei from subsequent growth. In other words, the slow exchange created a delay between the nucleation and growth phases, which improved the overall efficiency of the assembly process.
This finding has far-reaching implications for synthetic biology, as it provides a new strategy for designing compartmentalized systems that can efficiently assemble complex structures. By controlling the volume fractions and exchange rates between different compartments, researchers can optimize self-assembly processes to achieve specific outcomes.
The study’s authors also explored how this concept could be applied to larger spatially extended systems, such as surface patterns or membranes. They found that the optimal design parameters for these systems would depend on factors like the shape and size of the domains, as well as the rates of exchange between them.
This breakthrough has significant potential for advancing our understanding of self-assembly and its applications in synthetic biology. By developing new strategies for controlling and optimizing this process, researchers can create innovative solutions for medicine, energy, and other fields. As we continue to push the boundaries of what is possible with compartmentalized systems, we may uncover even more surprising insights into the intricate processes that govern life itself.
Cite this article: “Unlocking Efficient Self-Assembly in Compartmentalized Systems”, The Science Archive, 2025.
Self-Assembly, Compartmentalized Systems, Synthetic Biology, Biomolecular Systems, Slow Particle Exchange, Reaction Domains, Nucleation, Growth, Surface Patterns, Membranes.